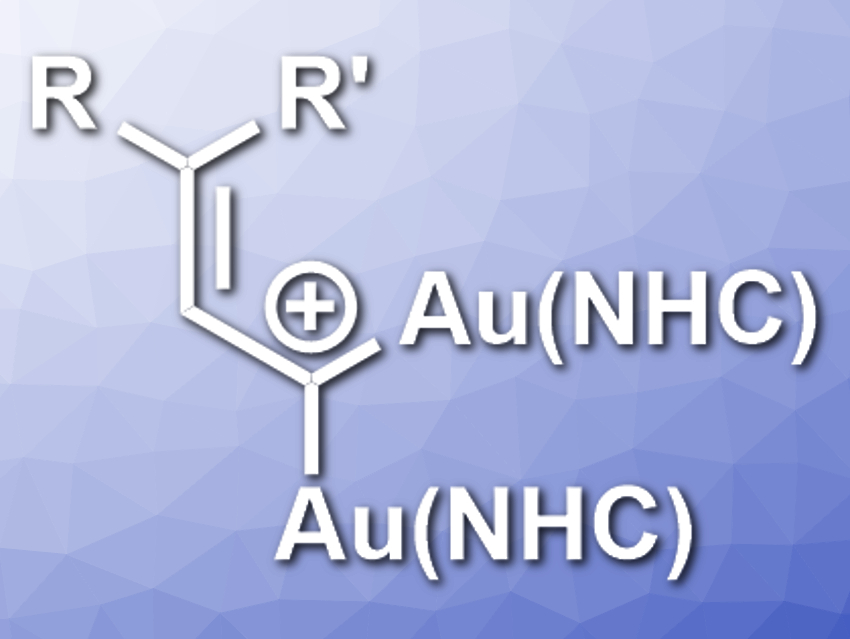
A. Stephen K. Hashmi and colleagues, Heidelberg University, Germany, have synthesized extremely reactive 1,1-digoldallylium complexes (pictured, NHC = N-heterocyclic carbene). The NHC ligands used were IPr (1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) derivatives. The team reacted (NHC)(cyclopropenyl)gold(I) complexes with cationic gold(I) complexes of the type [(IPr)Au]+ and obtained the 1,1-digoldallylium complexes in a ring-opening reaction.
The researchers confirmed the existence of these intermediates using UV−Vis−NIR spectrophotometry, NMR spectroscopy, and mass spectrometry. They were also able to capture the 1,1-digoldallylium complex with dimethylsulfoxide as a sulfoxonium species that is stable enough for X-ray crystallographic analysis. Density-functional theory (DFT) calculations were used to investigate the bonding situation in the 1,1-digoldallylium complex. The team found electron delocalization along the Au−C−Au region. The bonding can be described as a three-center−four-electron bond.
The 1,1-digoldallylium complexes can be considered a coordination compound of carbon with a previously unknown structure. According to the researchers, the reactivity of this kind of complex should be further investigated.
- 1,1-Digoldallylium Complexes: Diaurated Allylic Carbocations Indicate New Prospects of the Coordination Chemistry of Carbon,
Florian F. Mulks, Patrick W. Antoni, Jürgen H. Gross, Jürgen Graf, Frank Rominger, A. Stephen K. Hashmi,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.8b13395