Noble-metal-based catalysts are extremely useful for organic transformations. However, researchers are looking for more abundant and cost-effective alternatives. Main group elements such as the pnictogens (group 15 elements) are still only rarely used as alternative catalysts.
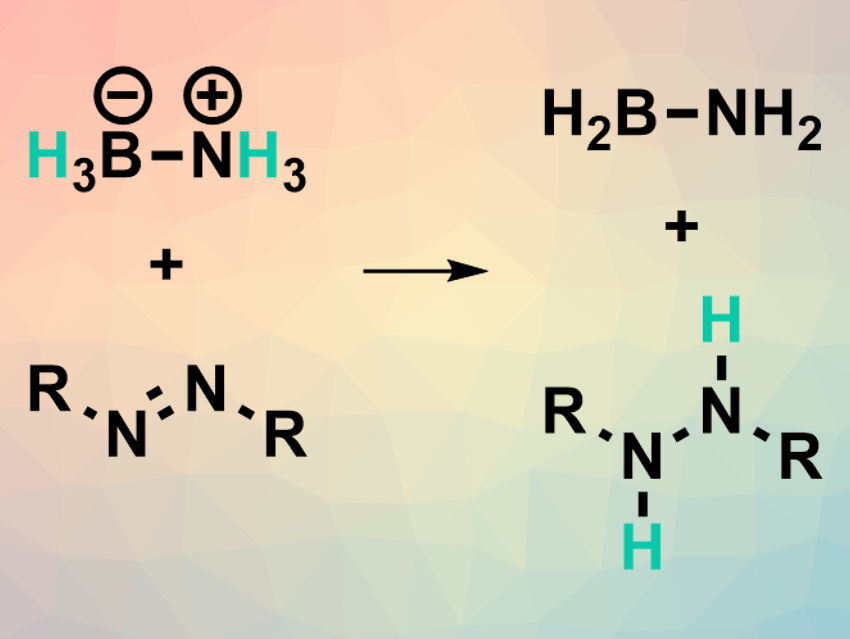
Josep Cornella, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed a transfer hydrogenation reaction (example pictured above) that uses a Bi(I) complex as the catalyst. The team converted a range of azoarenes to the corresponding hydrazines using ammonia-borane as a hydrogen source and a bismuthinidene complex (pictured right) as the catalyst in tetrahydrofurane (THF) at 35 °C. Under the same conditions, different nitroarenes were selectively converted to the corresponding N-arylhydroxylamines.
Bismuth is generally used in catalysis in the form of Bi(III) as a Lewis acid. In this transfer hydrogenation, however, the element acts as a redox-active catalyst, switching between Bi(I) and Bi(III). This makes the reaction a unique example of low-valent pnictogen catalysis.
- Bi(I)-Catalyzed Transfer-Hydrogenation with Ammonia-Borane,
Feng Wang, Oriol Planas, Josep Cornella,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b00594