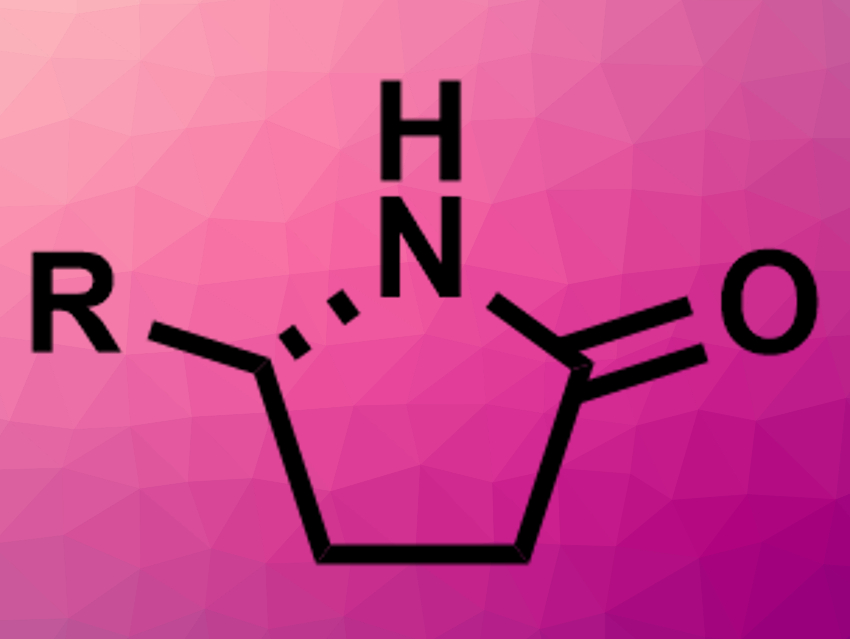
Lactams, i.e., cyclic amides, are important compounds in pharmaceutical chemistry. However, the enantioselective synthesis of, e.g., γ-lactams (pictured above) is still challenging.
Wing-Yiu Yu and colleagues, The Hong Kong Polytechnic University, have developed an enantioselective Ru-catalyzed intramolecular C–H amidation of 1,4,2-dioxazol-5-ones (pictured below), which gives γ-lactams. The team used RuCl(p-cymene)[(R,R)-Ts-dpen] (Ts = tosyl, dpen = diphenyl-1,2-diamine) as a chiral catalyst with an electron-withdrawing arylsulfonyl group. The dioxazolone reagents can be prepared from readily available carboxylic acids.
The reaction was performed using 10 mol % of the catalyst together with AgSbF6 in 1,2-dichloroethane (DCE) at 40 °C. The team obtained the desired γ-lactams in excellent yields and enantioselectivities (up to 97 % yield and 98 % ee).

- Ruthenium(II)-Catalyzed Enantioselective γ-Lactams Formation by Intramolecular C–H Amidation of 1,4,2-Dioxazol-5-ones,
Qi Xing, Chun-Ming Chan, Yiu-Wai Yeung, Wing-Yiu Yu,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b00535