N-heterocyclic carbenes (NHCs) can be used as organocatalysts. They can, e.g., promote radical reactions. Thiazolium salts, for example, are NHC precursors that can react with aldehydes in the presence of a base to form enamines. These enamines, so-called “Breslow intermediates”, can then perform a single-electron transfer (SET) to various electron acceptors and induce radical reactions.
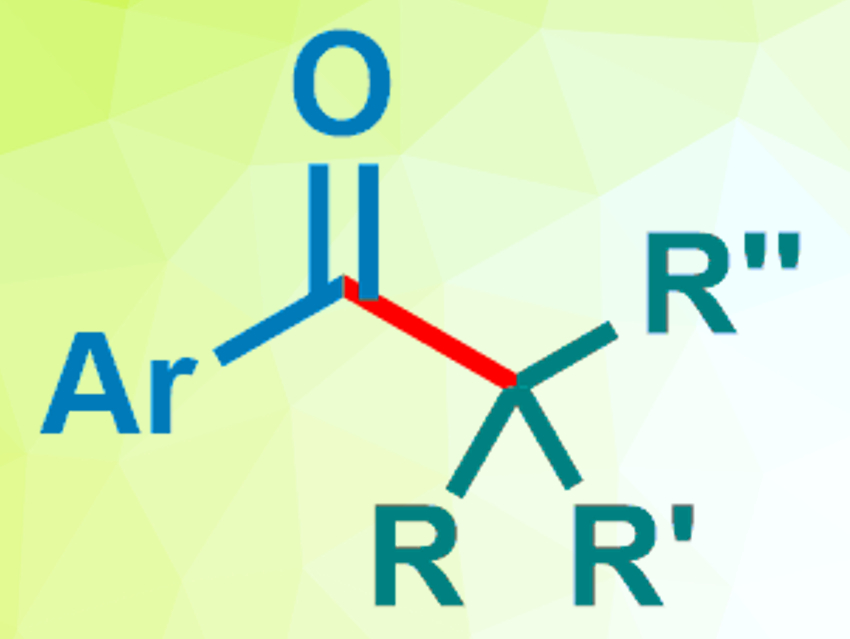
 Kazunori Nagao, Hirohisa Ohmiya, and colleagues, Kanazawa University, Japan, have used an N-2,6-diisopropylphenyl-substituted seven-membered ring-fused thiazolium salt as a precursor for an NHC catalyst (pictured right). This catalyst was used to promote the decarboxylative coupling between aryl aldehydes and redox-active esters derived from tertiary or secondary alkyl carboxylic acids (pictured below).
Kazunori Nagao, Hirohisa Ohmiya, and colleagues, Kanazawa University, Japan, have used an N-2,6-diisopropylphenyl-substituted seven-membered ring-fused thiazolium salt as a precursor for an NHC catalyst (pictured right). This catalyst was used to promote the decarboxylative coupling between aryl aldehydes and redox-active esters derived from tertiary or secondary alkyl carboxylic acids (pictured below).
The reaction was performed using 10 mol % of the catalyst precursor and Cs2CO3 as a base in dimethylsulfoxide (DMSO) at 60 °C. The desired products were obtained in good to excellent yields. The reaction proceeds under mild conditions and is transition-metal-free. The researchers propose a mechanism that involves an SET from an enolate form of the Breslow intermediate.

- N-Heterocyclic Carbene-Catalyzed Decarboxylative Alkylation of Aldehydes,
Takuya Ishii, Yuki Kakeno, Kazunori Nagao, Hirohisa Ohmiya,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b00880