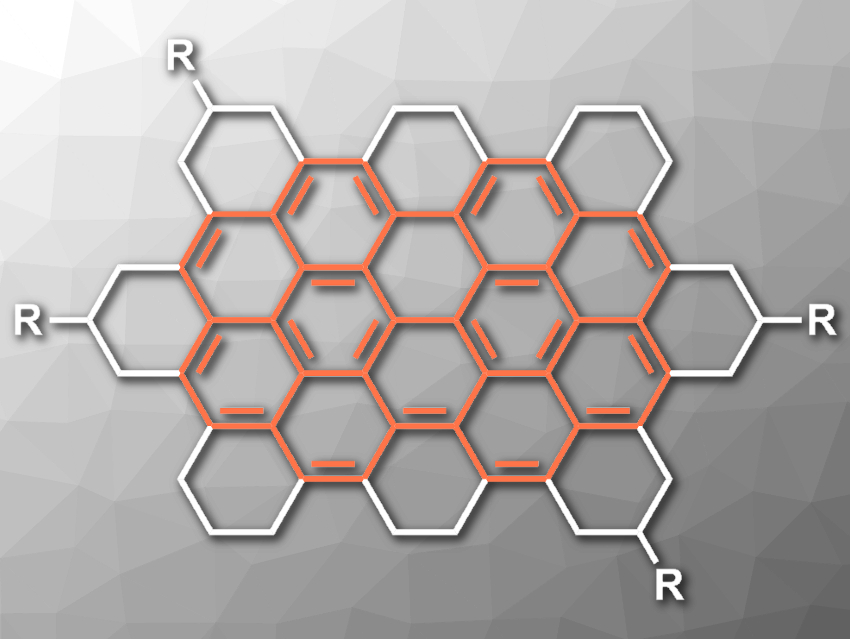
Circumbiphenyl (pictured in orange) is a polycyclic aromatic hydrocarbon (PAH) which consists of a biphenyl core inside an annulene ring. Derivatives of the compound and product mixtures containing circumbiphenyl have been prepared in the past. However, there had been no efficient synthesis of the actual aromatic structure of circumbiphenyl so far.
Akimitsu Narita, Max Planck Institute for Polymer Research, Mainz, Germany, and Okinawa Institute of Science and Technology Graduate University, Japan, Klaus Müllen, Max Planck Institute for Polymer Research and Johannes Gutenberg University Mainz, Germany, and colleagues have synthesized a peralkylated circumbiphenyl (pictured, R = n-C12H25) by the regioselective hydrogenation of a nanographene molecule with 60 sp2-hybridized carbon atoms. The hydrogenation reduces the size of the π-conjugated system (“π-truncation”).
The team performed the reaction using Pd/C as a catalyst in dry tetrahydrofuran (THF) as a solvent under 150 bar H2 pressure. The reaction mixture was kept at 120 °C for one week. The product was characterized using MALDI-TOF (matrix-assisted laser desorption/ionization–time of flight) mass spectrometry and NMR, infrared (IR), and Raman spectroscopy and confirmed to be the desired peralkylated circumbiphenyl. According to the researchers, the “π-truncation” approach could be useful for the synthesis of other hard-to-access aromatic structures.
- Regioselective Hydrogenation of a 60-Carbon Nanographene Molecule toward a Circumbiphenyl Core,
Xuelin Yao, Xiao-Ye Wang, Christopher Simpson, Giuseppe M. Paternò, Michele Guizzardi, Manfred Wagner, Giulio Cerullo, Francesco Scotognella, Mark D. Watson, Akimitsu Narita, Klaus Müllen,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b00384