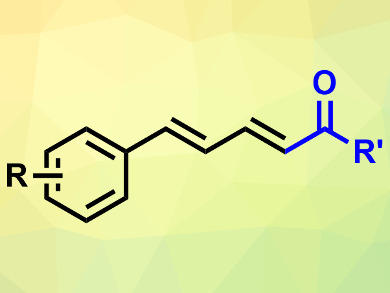
Sulfoxonium ylides can serve as carbene precursors in organic reactions. The can be used as safer alternatives to diazo compounds in this role.
Huanfeng Jiang, South China University of Technology, Guangzhou, and Lanzhou University, both China, and colleagues have developed a synthesis route to conjugated dienones (pictured) which is based on a palladium-catalyzed reaction between sulfoxonium ylides and allylarenes. The team reacted a variety of these substrates in the presence of Pd(OAc)2 as a catalyst, 2,6-dimethylcyclohexa-2,5-diene-1,4-dione (DMBQ) as an oxidant, and PPh3 as a ligand in dimethylsulfoxide (DMSO).
The desired conjugated dienones were obtained in moderate to good yields and with good regioselectivity. The reaction tolerates a range of functional groups and can be performed on a gram scale. According to the researchers, the method could be useful for the synthesis of bioactive molecules.
- Palladium-Catalyzed Oxidative Allylation of Sulfoxonium Ylides: Regioselective Synthesis of Conjugated Dienones,
Chunsheng Li, Meng Li, Wentao Zhong, Yangbin Jin, Jianxiao Li, Wanqing Wu, Huanfeng Jiang,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.8b03606