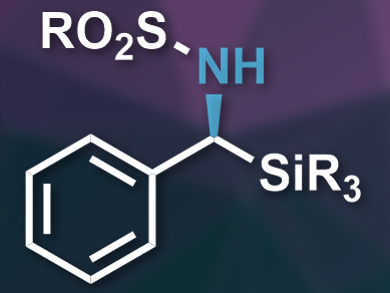
Chiral organosilicon compounds, such as α-aminosilanes, are useful in pharmaceutical chemistry. Existing methods for the synthesis of chiral α-aminosilanes usually require stoichiometric chiral reagents or other expensive reactants.
Zhenfeng Zhang, Wanbin Zhang, and colleagues, Shanghai Jiao Tong University, China, have developed the first asymmetric hydrogenation of N-sulfonyl arylsilylimines, which provides access to protected chiral α-aminosilanes (example pictured). The team used Pd(OCOCF3)2 as the catalyst, together with the chiral diphosphine ligand QuinoxP* or 2,3-bis(tert-butylmethylphosphino)quinoxaline. This allowed them to convert a range of N-sulfonyl arylsilylimines into the corresponding amines under an H2 atmosphere.
The reaction proceeds with high yields and excellent enantioselectivities. The sulfonyl protecting group (RO2S–) can be removed by converting it to a tert-butoxycarbonyl (Boc) group using di-tert-butyl dicarbonate (Boc2O). The Boc group is then removed using chlorotrimethylsilane (TMSCl) to give the free α-aminosilanes. According to the researchers, these products can act as mimics of natural α-amino acids in biologically active compounds.
- Synthesis of Chiral α-Aminosilanes through Palladium-Catalyzed Asymmetric Hydrogenation of Silylimines,
Dongyang Fan, Yang Liu, Jia Jia, Zhenfeng Zhang, Yangang Liu, Wanbin Zhang,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.8b04073