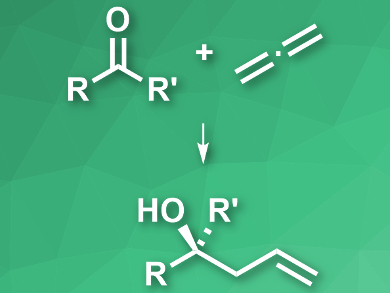
Homoallylic alcohols are useful intermediates in organic synthesis. They can be prepared by the allylation of ketones. However, stereoselective variations of this reaction usually require stoichiometric amounts of allylmetal reagents. Replacing these reagents would make the reaction greener and more cost-effective.
Stephen L. Buchwald and colleagues, Massachusetts Institute of Technology (MIT), Cambridge, USA, have developed a protocol for the enantioselective allylation of ketones using allene (pictured). Allene gas is produced as a byproduct in petroleum cracking on a scale of a million metric tons, but is not commonly used as a chemical feedstock. The team combined allene with (MeO)2MeSiH as a reductant, Cu(OAc)2 as a catalyst, asymmetric phosphines as ligands, and a range of ketones to give the desired homoallylic alcohols in high yields and optical purities.
The reaction is highly selective and can even be performed with mixtures of allene, propylene, and methylacetylene obtained directly from cracking. The silane can also be replaced with the inexpensive polymer polymethylhydrosiloxane (PMHS), a waste product of the silicone industry. According to the researchers, allene gas could serve as a versatile and economical reagent in a variety of other reactions.
- Enantioselective Allylation Using Allene, a Petroleum Cracking Byproduct,
Richard Y. Liu, Yujing Zhou, Yang Yang, Stephen L. Buchwald,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.8b13907