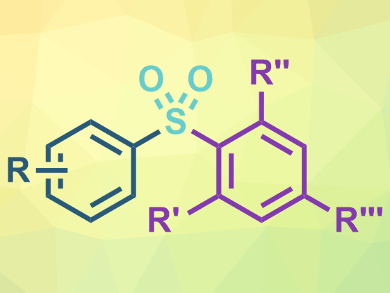
Diarylsulfones occur, e.g., in drugs and biologically active compounds. They can be prepared using transition-metal-catalyzed three-component coupling reactions. However, known methods for this type of reaction do not work well with bulky, ortho-substituted aryl groups (pictured in purple).
Tao Tu, Fudan University and Chinese Academy of Sciences, both Shanghai, China, and colleagues have developed a one-step gold(I)-catalyzed protocol to access ortho-substituted diarylsulfones. The team used aryl boronic acids, potassium metabisulfite (K2S2O5), and diaryliodonium salts as substrates. An N-heterocyclic carbene (NHC)-gold(I) complex was used as the catalyst, Na2CO3 as a base, and acetonitrile as the solvent. The reaction was performed at 100 °C and the desired products were obtained in good to excellent yields (example pictured).
The reaction selectively transfers the more sterically demanding aryl groups of the diaryliodonium salts. The team proposes that this is due to the fact that the intermediate Ar+ ions of bulky, electron-rich aryl groups are more stable than those of smaller, electron-poor aryl groups
- Selective Synthesis of ortho-Substituted Diarylsulfones by Using NHC-Au Catalysts under Mild Conditions,
Haibo Zhu, Yajing Shen, Daheng Wen, Zhang-Gao Le, Tao Tu,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.8b03957