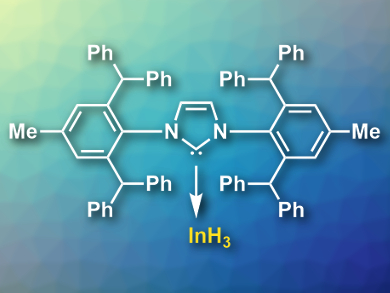
Complexes of group 3 hydrides such as alane (AlH3) and gallane (GaH3) are useful, e.g., as reducing agents in organic chemistry. In contrast, only few examples of indane (InH3) complexes are known due to their inherent instability. Bulky ligands can be used to stabilize InH3 and protect it from decomposition reactions. However, if the ligands have alkyl groups close to the In atom, this can speed up decomposition due to C–H activation in the alkyl groups.
Alasdair I. McKay and colleagues, University of New South Wales, Sydney, Australia, have synthesized an exceptionally stable N-heterocyclic carbene (NHC) indane complex, [InH3(IPr*)] (pictured, iPr* = 1,3-bis(2,6-bis(diphenylmethyl)-4-methylphenyl)imidazol-2-ylidene). The team prepared the complex using a direct reaction of IPr* with LiInH4.
The complex is stable in crystalline form at room temperature in air for an indefinite period and decomposes at 182 °C under argon. It does not decompose in solution at 0 °C. The team attributes this stability to a lack of alkyl groups in close proximity to In, as well as to the steric bulk of the NHC ligand, which stabilizes the complex kinetically.
- An exceptionally stable NHC complex of indane (InH3),
Anthony R. Leverett, Marcus L. Cole, Alasdair I. McKay,
Dalton Trans. 2019, 48, 1591–1594.
https://doi.org/10.1039/c8dt04956d