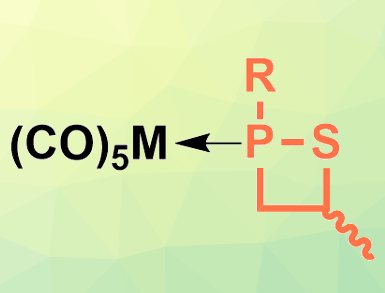1,2-Oxaphosphetanes, i.e., four-membered rings containing neighboring P(V) and O atoms, are well known. 1,2-thiaphosphetanes containing P(V) and S, in contrast, are less well studied, and their P(III) derivatives have been unknown so far. Such four-membered rings could be useful ligands due to their ring strain and reactivity.
Arturo Espinosa Ferao, Universidad de Murcia, Spain, Rainer Streubel, University of Bonn, Germany, and colleagues have synthesized the first P(III) 1,2-thiaphosphetane complexes with group 6 metals (pictured, R = CPh3), as well as the free ligand. The team reacted Li/Cl phosphinidenoid complexes of the metals Cr, Mo, and W with propylene sulfide to obtain the desired 1,2-thiaphosphetane complexes as a mixture of two stereoisomers. The free 1,2-thiaphosphetane ligand (pictured in orange) was prepared from the molybdenum complex using ligand exchange with 1,2-bis(diphenylphosphino)ethane (dppe) in toluene.
The researchers confirmed the structure of the products using 31P NMR spectroscopy and X-ray diffraction measurements, as well as theoretical calculations. The electronic properties of the free ligand, particularly its small HOMO–LUMO gap, could make it useful for redox-cycling organocatalysts that switch between P(III) and P(V) species.
- Synthesis of free and ligated 1,2-thiaphosphetanes – expanding the pool of strained P-ligands,
Andreas Wolfgang Kyri, Florian Gleim, David Becker, Gregor Schnakenburg, Arturo Espinosa Ferao, Rainer Streubel,
Chem. Commun. 2019.
https://doi.org/10.1039/c8cc09892a