Using alkenes in carbon–carbon bond formation reactions is common in organic chemistry. However, it can be difficult to perform such reactions using large, unactivated alkenes without any directing groups.
Ryo Takeuchi and colleagues, Aoyama Gakuin University, Sagamihara, Japan, have developed an intermolecular hydroalkylation of unactivated terminal alkenes, catalyzed by a cationic iridium complex. The team used [Ir(cod)2]SbF6 as a catalyst (cod = 1,5-cyclooctadiene) and 1,2-dichloroethane (DCE) as a solvent to react a range of terminal alkenes, e.g., 1-octene, with different 1,3-diketones, e.g., 2,4-pentadione.
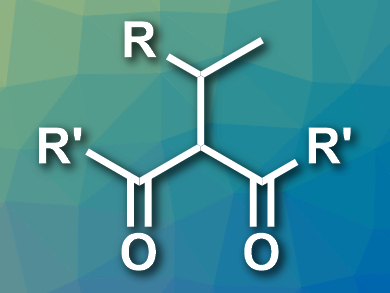
The desired Markovnikov-type products (example pictured) were obtained in good yields. These diketone products are useful starting materials for the synthesis of substituted heterocyclic compounds. According to the researchers, the developed reaction provides a new approach for extending the carbon chain of unactivated aliphatic terminal alkenes.
- Cationic Iridium Complex-Catalyzed Intermolecular Hydroalkylation of Unactivated Alkenes with 1,3-Diketones,
Ryo Takeuchi, Jun Sagawa, Masaki Fujii,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.8b03975