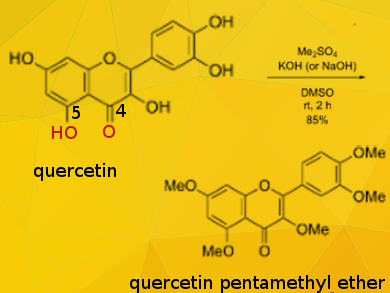
Quercetin (3,5,7,3′,4′-pentahydroxyflavone) is a plant pigment (flavonoid). Among the five hydroxy groups of quercetin, the OH group at position 5 is the most resistant to methylation due to its strong intramolecular hydrogen bonding with the carbonyl group at position 4. Thus, it is generally difficult to synthesize the pentamethyl ether efficiently by conventional methylation (using MeI and K2CO3 in dimethylformamide (DMF), reflux, 23 hours).
Tsutomu Ishikawa, Tokiwa Phytochemical Co. Ltd., Chiba, Japan, and colleagues have developed a simple and effective per-O-methylation of quercetin with dimethyl sulfate as methylating agent in potassium (or sodium) hydroxide/dimethyl sulfoxide (DMSO) at room temperature for about 2 hours, affording quercetin pentamethyl ether (QPE) quantitatively as a single product.
When methyl iodide was used instead of dimethyl sulfate, 6-methyl-3, 5, 7, 3′, 4′-pantamethylquercetin was also formed as a byproduct. The experimental results and computational studies allowed the researchers to deduce the reactivity order of the five OH functions on quercetin to be 7 ≈ 4′ > 3 > 3′ > 5.
- A simple and effective preparation of quercetin pentamethyl ether from quercetin,
Jin Tatsuzaki, Tomohiko Ohwada, Yuko Otani, Reiko Inagi, Tsutomu Ishikawa,
Beilstein J. Org. Chem. 2018, 14, 3112–3121.
https://doi.org/10.3762/bjoc.14.291