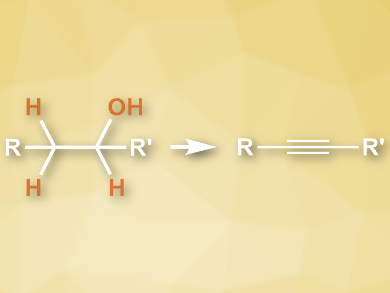
Alkynes are useful reagents as well as products in organic synthesis. They can be synthesized from low-cost, readily available alcohols. However, these processes usually require two steps: the alcohols are first oxidized to aldehydes or ketones, which are then converted to alkynes.
Hua-Li Qin, Wuhan University of Technology, China, and colleagues have developed a direct synthesis of alkynes from alcohols, using a dehydration and dehydrogenation process which is mediated by sulfuryl fluoride (SO2F2). The team used SO2F2 to activate dimethyl sulfoxide (DMSO) for alcohol oxidation. The resulting carbonyl intermediates further react with SO2F2 in situ to give vinyl fluorosulfates. K2CO3 and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) were used as bases. CsF was used to promote the final step, the elimination of a fluorosulfonic ester. DMSO also serves a the solvent for the reaction.
The desired alkynes were obtained in high yields and with sufficient purity for further functionalization in one-pot reactions. The reaction protocol works well for a variety of homobenzylic and aliphatic alcohols. The reagents are readily available at low costs and the reaction proceeds under mild conditions.
- SO2F2-Mediated Oxidative Dehydrogenation and Dehydration of Alcohols to Alkynes,
Gao-Feng Zha, Wan-Yin Fang, You-Gui Li, Jing Leng, Xing Chen, Hua-Li Qin,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b10069