Aliphatic amines are an important class of organic compounds, used widely in research and industry. There is a range of synthesis methods for amines on primary carbon centers. However, a wider selection of methods that lead to amines on secondary or tertiary carbon centers would be useful.
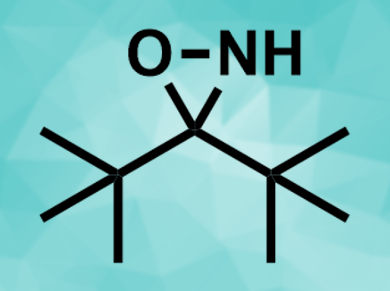
László Kürti, Rice University BioScience Research Collaborative, Houston, TTX, USA, and colleagues have developed a protocol for the electrophilic amination of primary, secondary, and tertiary organometallic substrates, i.e., alkylmagnesium- and alkylzinc halides. The team used a bench-stable NH-oxaziridine (pictured) as a nitrogen-transfer agent. The reaction proceeds in diethyl ether at room temperature.
The developed reaction gives the desired amines in good to excellent yields. It is simple to perform, needs no transition metal catalysts, and tolerates both electron-donating and electron-withdrawing functional groups.
- Direct Primary Amination of Alkylmetals with NH-Oxaziridine,
Nicole Erin Behnke, Russell Kielawa, Doo-Hyun Kwon, Daniel H. Ess, László Kürti,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03734