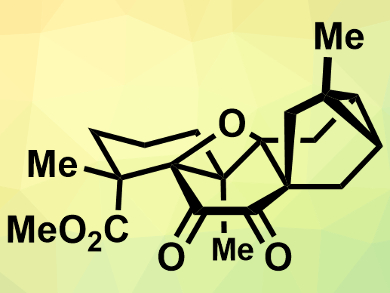
Oxetanes, i.e., four-membered rings containing an oxygen atom, are found in some natural products, such as the anticancer compound taxol. Another example of an oxetane, embedded in a pentacyclic carbon skeleton, is found in the natural product (−)-mitrephorone A. This compound was isolated from the Bornean shrub Mitrephora glabra, and so far, it had not been synthesized. The compound has antimicrobial and cytotoxic properties.
Erick M. Carreira and colleagues, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, have performed the first total synthesis of (−)-mitrephorone A. The team used several Diels-Alder reactions to build the polycyclic framework. The α-diketone was prepared by a Riley oxidation using SeO2. The oxetane ring is fully substituted and, thus, synthetically challenging. It was formed in a final step from the corresponding hydroxy diosphenol using an oxidative cyclization mediated by hypervalent iodine.
The synthesis is enantioselective and the product was characterized using 1H- and 13C NMR spectroscopy, infrared (IR) spectroscopy, polarimetry, and mass spectrometry (MS). The analytical data were in agreement with those reported for the natural product. According to the researchers, the formation reaction of the oxetane ring is unprecedented and extends the organic chemist’s toolbox for the synthesis of oxetanes.
- Total Synthesis of (–)-Mitrephorone A,
Matthieu J. R. Richter, Michael Schneider, Marco Brandstätter, Simon Krautwald, Erick M. Carreira,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b09685