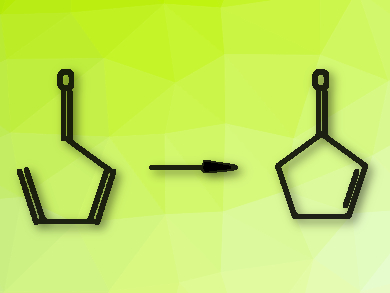
Cyclopentenones (pictured right) occur in important natural products and pharmaceutically active compounds. They can be synthesized from acyclic precursors, but the available reactions need either toxic reagents, harsh conditions, or difficult-to-prepare substrates.
Vincent Gandon, Institut de Chimie Moleculaire et des Matériaux d’Orsay (ICMMO), France, and Ecole Polytechnique, Université Paris-Saclay, France, Martín J. Riveira, Universidad Nacional de Rosario-CONICET, Argentina, and colleagues have developed a simple, green, and efficient synthesis of 2‑cyclopentenones. The team used readily available all-trans 2,4-dienals (pictured left) as starting materials, iodine as a catalyst, and ethyl acetate as a solvent. The reaction was performed at 120 °C and gave the desired 2‑cyclopentenones in good to excellent yields.
The cyclization is a so-called iso-Nazarov reaction. According to the researchers, it proceeds via an unexpected concerted mechanism, in which iodine activates the carbonyl group. This was supported by density functional theory (DFT) calculations. The reaction tolerates a range of substituents on the dienal and offers a new path to useful 2-cyclopentenones.
- Iodine-Catalyzed Iso-Nazarov Cyclization of Conjugated Dienals for the Synthesis of 2-Cyclopentenones,
Lucía A. Marsili, Jorgelina L. Pergomet, Vincent Gandon, Martín J. Riveira,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03229




And cyclohexenone can be synthesized in the same way!