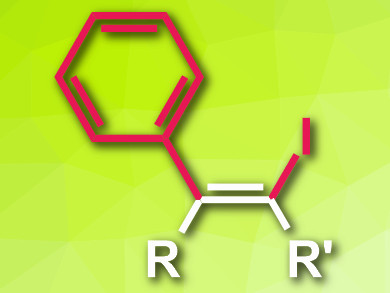
Alkenyl halides are useful synthetic intermediates with a C=C double bond and a halogen substituent. One approach for their synthesis is the insertion of an alkyne into the carbon–halogen bond of an organohalogen compound, catalyzed by transition-metal complexes. However, this generally requires bulky ligands, which makes the reaction slow and usually limits it to intramolecular cases.
Takuya Kurahashi, Seijiro Matsubara, and colleagues, Kyoto University, Japan, have developed an intermolecular, nickel-catalyzed reaction of alkynes with aryl iodides that gives alkenyl iodides (pictured). This reaction proceeds via a radical mechanism and avoids the need for bulky ligands. The team used a range of aryl iodides and substituted alkynes as substrates, Ni(cod)2 as a catalyst (cod = 1,5-cyclooctadiene), 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbdp) as a ligand, and toluene as solvent.
The reaction gives the desired alkenyl iodides in high yields. The proposed mechanism involves the oxidative addition of the aryl iodide to the nickel complex, followed by the homolysis of the resulting unstable Ni–C bond, which generates a radical. This radical adds to the alkyne’s triple bond, recombines with the nickel iodide complex, and the final product is released via a reductive elimination.
- Nickel-catalyzed intermolecular carboiodination of alkynes with aryl iodides,
Toshifumi Takahashi, Daiki Kuroda, Toru Kuwano, Yuji Yoshida, Takuya Kurahashi, Seijiro Matsubara,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc07560c