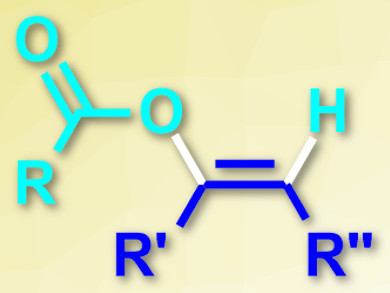
Enol esters (pictured) are useful intermediates for organic synthesis. An atom-economical way to prepare them is the addition of carboxylic acids to alkynes, catalyzed by transition metals. However, this usually requires either toxic or expensive metals.
Jia-Feng Chen and Changkun Li, Shanghai Jiao Tong University, China, have developed a cobalt-catalyzed regio- and stereoselective addition of carboxylic acids to alkynes which gives enol esters. The team used Co(BF4)2 as a catalyst together with a tridentate organophosphorus ligand (triphos) and Zn as a reductant. The reactions were performed in acetonitrile at room temperature.
The desired enol esters were obtained in high yields and stereoselectivities. The method tolerates both terminal and internal alkynes. In contrast to previously developed syntheses, neither the cobalt salt nor the ligand is expensive. According to the researchers, the developed cobalt/triphos catalyst complex may also be useful for other reactions.
- Enol Ester Synthesis via Cobalt-Catalyzed Regio- and Stereoselective Addition of Carboxylic Acids to Alkynes,
Jia-Feng Chen, Changkun Li,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02824