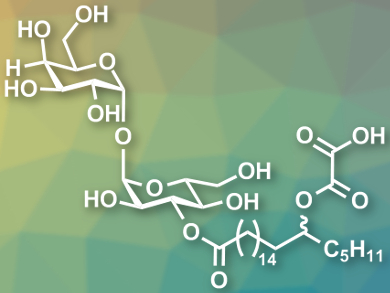
The influenza virus periodically causes pandemics. New vaccines and treatments are needed to combat this potentially life-threatening disease. The glycolipids emmyguyacins A (pictured) and B were isolated from a fungus. The compounds, which only differ in the configuration at one stereocenter, can inhibit the replication of the influenza A virus. Four types of influenza viruses are known; three of them affect people, Type A, Type B, and Type C.
Suvarn S. Kulkarni and colleagues, Indian Institute of Technology Bombay, Mumbai, India, have performed the first total synthesis of emmyguyacins A and B. The team first tackled the lipid unit of the compounds, a 17-oxalyloxydocosanoic acid. They started from the commercially available (S)-epichlorohydrin and used a reaction sequence of the type Grignard addition–epoxidation–Grignard addition, followed by a Julia–Kocienski olefination, to give the desired fatty acid derivatives.
The team then started the synthesis of the emmyguyacins from trehalose, which was protected in several positions, then combined with the prepared fatty acid derivative and deprotected to give the desoxylate emmyguacin derivatives. The oxalate ester group (pictured on the far right) was then introduced in a final step.
The desired emmyguyacins were obtained in pure enantiomeric forms in overall yields of 2.7 % for emmyguyacin A and 12.2 % for emmyguyacin B. The synthesis requires a total of 24 steps for emmyguyacin A and 18 steps for emmyguyacin B. According to the researchers, this result should allow the compounds to be tested in influenza virus inhibition studies.
- Total Synthesis of Emmyguyacins A and B, Potential Fusion Inhibitors of Influenza Virus,
Santanu Jana, Vikram A. Sarpe, Suvarn S. Kulkarni,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03073