Aromatic molecules usually feature a ring of overlapping π-orbitals with 4n+2 electrons (according to the Hückel rule). However, orbitals with other symmetries, such as σ-orbitals, can also form aromatic systems. The existence of “double aromaticity”, in which one molecule features both σ- and π-aromaticity, has been predicted theoretically. However, examples of this are rare and no bench-stable doubly aromatic compound had been characterized so far.
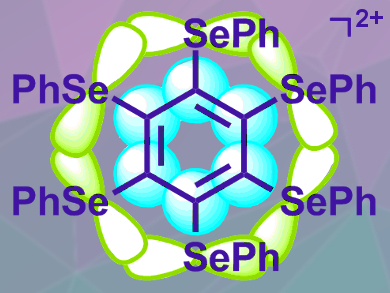
Masaichi Saito, Saitama University, Japan, and colleagues have synthesized and isolated the hexakis(phenylselenyl)benzene dication (pictured), which shows double aromaticity. The team synthesized the cation via the two-electron oxidation of its neutral precursor using nitrosonium hexafluoroantimonate ([NO]SbF6). The desired hexafluoroantimonate salt was obtained in a yield of 73 %.
The cation has an inner benzene ring, with six electrons in a ring of π-orbitals (pictured in blue), and an outer selenium six-ring, with ten electrons in a ring of σ-orbitals (pictured in green). Both follow the Hückel rule. X-ray diffraction showed that the distances between adjacent selenium atoms are similar, which would be expected for an aromatic system. NMR measurements provided evidence for an induced σ-ring current in the Se6 group, which also is consistent with aromaticity.
- Double aromaticity arising from σ- and π-rings,
Shunsuke Furukawa, Masahiro Fujita, Yoshihiko Kanatomi, Mao Minoura, Miho Hatanaka, Keiji Morokuma, Kazuya Ishimura, Masaichi Saito,
Commun. Chem. 2018.
https://doi.org/10.1038/s42004-018-0057-4