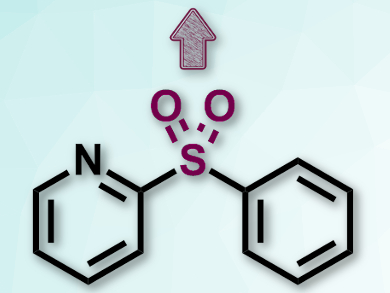
Organosulfur compounds are useful reagents in organic synthesis. Sulfones are particularly interesting due to the leaving group character of sulfonyl. Dialkyl sulfones, for example, can be converted to alkenes via a loss of SO2.
Hideki Yorimitsu and colleagues, Kyoto University, Japan, have developed an intramolecular desulfitative coupling that converts diaryl sulfones to the corresponding biaryls via a catalytic elimination of SO2. The team used a range of aryl 2-quinolyl sulfones (example pictured) as substrates, Ni(cod)2 (cod = 1,5-cyclooctadiene ) as a catalyst, an N-heterocyclic carbene as a ligand, and Mg shavings as a reducing additive.
The desired diaryl products were obtained in moderate to good yields. The proposed catalytic cycle involves the formation of a Ni(0)–NHC complex, which cleaves one C–SO2 bond to give arylnickel(II) sulfinates. SO2 is then eliminated from these intermediates, leading to diarylnickel(II) species. A final reductive elimination gives the desired biaryl compounds. The developed approach can also be used for the conversion of alkenyl aryl sulfones to alkenyl arenes.
- Intramolecular Desulfitative Coupling: Nickel-Catalyzed Transformation of Diaryl Sulfones into Biaryls via Extrusion of SO2,
Fumiya Takahashi, Keisuke Nogi, Hideki Yorimitsu,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02972