Conjugated dienes are useful reactants in organic chemistry. However, achieving chemo- or regioselectivity in reactions involving dienes can sometimes be challenging. While “linear” 1,3-dienes have been extensively used, there is only a limited number of useful reactions starting from “branched” 2-substituted 1,3-dienes.
Daniele Fiorito and Clément Mazet, University of Geneva, Switzerland, have developed an iridium-catalyzed, 4,3-selective hydroboration of 2-substituted 1,3-dienes. The team used a variety of alkyl-, aryl-, or heteroaryl-substituted dienes as substrates, pinacolborane (HBpin) as the reaction partner, [IrCl(COD)]2 as a catalyst, 1,2-bis(diphenylphosphino)ethane (dppe) as a ligand, and tetrahydrofuran (THF) as the solvent.
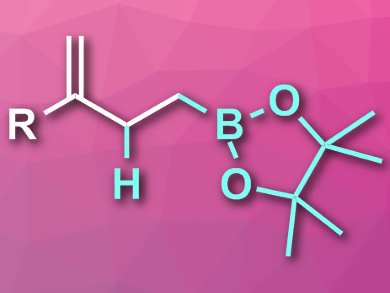
The reaction proceeds at room temperature and gives the desired homoallylic boronates (pictured) in isolated yields of 55–82 % and with high chemo- and regioselectivity. A wide range of functional groups is tolerated. The prepared homoallylic boronates can be further converted, e.g., by Zweifel olefinations, additional Ir-catalyzed hydroborations, or Pd-catalyzed Suzuki cross-couplings. Thus, according to the researchers, these building blocks could have widespread applications in synthesis.
- Ir-Catalyzed Selective Hydroboration of 2-Substituted 1,3-Dienes: A General Method to Access Homoallylic Boronates,
Daniele Fiorito, Clément Mazet,
ACS Catal. 2018.
https://doi.org/10.1021/acscatal.8b02334