Halogen bonds are non-covalent interactions between the electrophilic region of a halogen atom and an electron donor. This type of interaction can be useful for crystal engineering, i.e., the design of crystalline materials with specific, desirable properties. This is due to the fact that the interactions are relatively strong and highly directional: halogen bonds tend to form angles close to 180 °. Phosphines are commonly used as electron donors in chemistry. However, there are only very few examples of phosphines as halogen-bond acceptors.
David L. Bryce and colleagues, University of Ottawa, Canada, have synthesized a co-crystal that features phosphines as halogen-bond acceptors. The team combined 1,3,5-trifluoro-2,4,6-triiodobenzene (sym-C6F3I3, a commonly used halogen-bond donor) with triphenylphosphine (PPh3) in diethyl ether. Co-crystals were formed via slow evaporation of the solvent and characterized using X-ray diffraction. The formed co-crystals belong to the triclinic crystal system and the P1 space group.
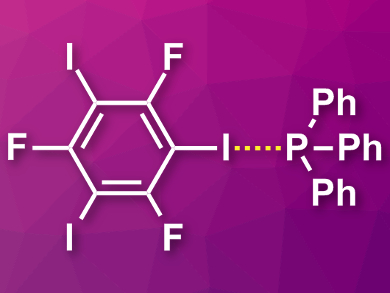
The team found a halogen bond between the iodine in sym-C6F3I3 and the phosphorus atom (pictured). The bond angle is 165 °. This falls within the typical halogen-bonding-angle range. The I–P distance is 3.375 Å. Using 31P NMR, it was shown that the P–I interaction persists in solution. According to the researchers, the results could open up new directions in crystal engineering and the design of materials.
- A rare example of a phosphine as a halogen bond acceptor,
Yijue Xu, Jasmine Huang, Bulat Gabidullin, David L. Bryce,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc06019c