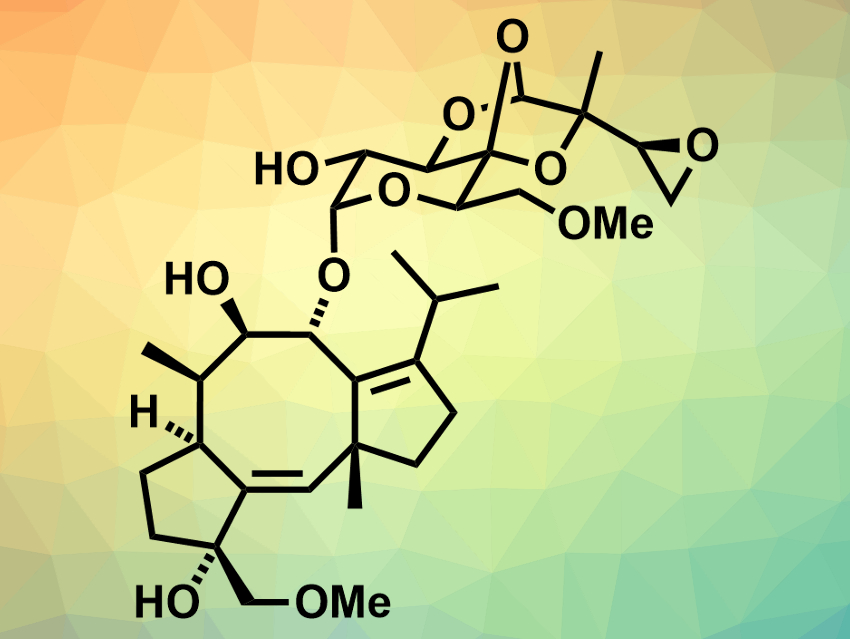
Cotylenin A (pictured) was originally isolated from a fungus. It is bioactive and can act as a plant growth regulator and an anti-cancer agent. While its absolute structure has been determined using X-ray crystallography, no total synthesis of cotylenin A had been reported so far. Only the total synthesis of the related cotylenol, which misses the glycosyl unit, had been achieved [1].
Masahisa Nakada, Waseda University, Tokyo, Japan, and colleagues have performed a convergent enantioselective total synthesis of cotylenin A. The team prepared the cyclopentane fragment using a catalytic asymmetric intramolecular cyclopropanation and the cyclopentene fragment via a modified acyl radical cyclization. These two fragments were then combined using an Utimoto coupling reaction and a palladium-mediated cyclization to give the 5-8-5 carbocyclic ring system. The sugar unit was prepared from an epoxy aldehyde and a glucose-derived α-hydroxy ketone. A glycosylation using Wan’s protocol was then performed the combine the 5-8-5 ring system with the sugar subunit and obtain the desired product.
Overall, the synthesis involved 25 steps (longest linear sequence). Optimization of the synthesis could lead to amounts of cotylenin A that are suitable for biological studies.
- Enantioselective Total Synthesis of Cotylenin A,
Masahiro Uwamori, Ryunosuke Osada, Ryoji Sugiyama, Kotaro Nagatani, Masahisa Nakada,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c01774
Reference
- [1] Total Synthesis of (−)-Cotylenol, a Fungal Metabolite Having a Leaf Growth Activity,
Hiroaki Okamoto, Hiroaki Arita, Nobuo Kato, Hitoshi Takeshita,
Chem. Lett. 1994, 23, 2335–2338.
https://doi.org/10.1246/cl.1994.2335