The alkaline earth elements have two valence electrons in an ns2 configuration. Thus, these main-group metals typically act very differently from transition metals and usually form divalent species. It has, however, been suggested that the heavy element barium can sometimes use its 5d orbitals in chemical bonding.
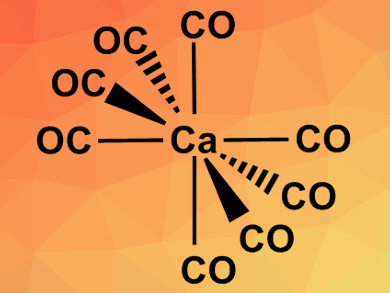
Inspired by this idea, Mingfei Zhou, Fudan University, Shanghai, China, Gernot Frenking, Nanjing Tech University, China, and University of Marburg, Germany, have tried to find evidence for the existence of the octacarbonyl complex Ba(CO)8. The team not only found the barium complex, but also the analogous calcium and strontium compounds, i.e., Ca(CO)8 and Sr(CO)8. The complexes were prepared in solid neon from the respective metal atoms (evaporated by a pulsed laser) and CO. The reactions were monitored using Fourier transform infrared (FT-IR) spectroscopy. Quantum chemical calculations were used to assign the spectral bands and to study the bonding situation in the complexes.
The results showed that not only barium but also strontium and calcium can use their (n−1)d orbitals in chemical bonding. The resulting complexes follow the 18-electron rule usually used for transition metals. According to the researchers, the capacity of the heavier alkaline earth elements to behave like transition metals should be considered in future studies.
- Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals,
Xuan Wu, Lili Zhao, Jiaye Jin, Sudip Pan, Wei Li, Xiaoyang Jin, Guanjun Wang, Mingfei Zhou, Gernot Frenking,
Science 2018, 361, 912–916.
https://doi.org/10.1126/science.aau0839