Nitrile ylides are useful synthetic intermediates. They are readily accessible and act as 1,3-dipoles in cycloadditions. While intramoleceular cyclizations using nitrile ylides are well developed, analogous intermolecular reactions are rare. Specifically, there had not been a reliable method to trap nitrile ylides with triple-bonded molecules so far.
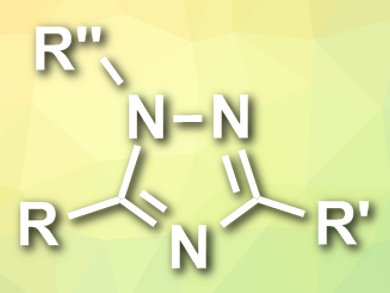
Xiaoguang Bao, Xiaobing Wan, and colleagues, Soochow University, Suzhou, China, have developed a (3+2) cycloaddition of nitrile ylides with diazonium salts for the synthesis of substituted 1,2,4-triazoles (pictured). The team combined different aryldiazonium tetrafluoroborates with various nitriles and diazo acetates in the presence of CuBr as a catalyst and Li2CO3 as a base at 40°C. The desired substituted 1,2,4-triazoles were obtained in moderate to high yields.
The proposed reaction mechanism starts with the formation of a carbenoid copper complex from the diazo compound with a loss of N2. The intermediate nitrile ylide species is then formed by a nucleophilic attack of the nitrile on the carbenoid complex. Finally, the nitrile ylide is trapped by the diazonium salt in a (3+2) cycloaddition reaction, followed by isomerization to give the triazole product. According to the researchers, the developed method offers a simple and efficient strategy to construct structurally diverse 1,2,4-triazoles from readily available starting materials in one step and under mild conditions.
- [3 + 2] Cycloaddition of Nitrile Ylides with Diazonium Salts: Copper-Catalyzed One-Pot Synthesis of Fully Substituted 1,2,4-Triazoles,
Huihuang Li, Xueli Wu, Weiwei Hao, Haiyan Li, Yanwei Zhao, Yaxiong Wang, Pengcheng Lian, Yonggao Zheng, Xiaoguang Bao, Xiaobing Wan,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02172