Fluorine-18 is a radionuclide that is commonly used in tracer molecules for positron emission tomography (PET) scans. The isotope can be produced from oxygen-18 by irradiation of oxygen-18-enriched water. However, this produces strongly hydrated 18F–, which is unreactive and cannot be directly used as a nucleophile in the synthesis of tracers. The usual approach to “drying” 18F– involves time-consuming steps (e.g., repeated distillations) and leads to an overall loss of radioactivity.
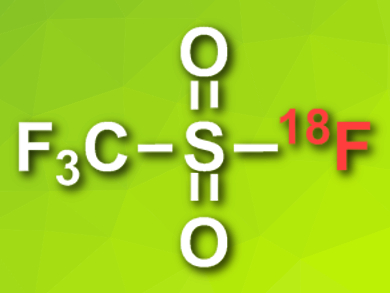
Anna Pees, Vrije Universiteit Medical Center, Amsterdam, The Netherlands, and colleagues have developed a faster, simpler process to generate “dry” 18F–. The team first trapped the prepared 18F– on an anion-exchange resin in a column. The fluoride is then washed from the column using a potassium sulfate solution. The 18F–-containing eluent was then added to a solution of bistriflate in dimethylformamide (DMF) to give gaseous [18F]triflyl fluoride (pictured). This gas can be trapped in a solution of a base (KHCO3) and a cryptand ligand in acetonitrile to provide “dry”, reactive 18F– in radiochemical yields of over 90 %. The process only takes five minutes.
The team confirmed the reactivity of the produced 18F– by using it in the synthesis of the PET tracers [18F]fluoroestradiol (used for breast cancer imaging) and O-2-[18F]fluoroethyl-L-tyrosine (a radiolabelled amino acid). They achieved good isolated radiochemical yields. According to the researchers, the developed method could be broadly applied to provide 18F– for radiofluorination reactions.
- Fast and reliable generation of [18F]triflyl fluoride, a gaseous [18F]fluoride source,
A. Pees, C. Sewing, M. J. W. D. Vosjan, V. Tadino, J. D. M. Herscheid, A. D. Windhorst, D. J. Vugts,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc03206h