Trifluoromethyl groups can be useful, e.g., in pharmaceutical chemistry to tune the properties of drug candidates. The trifluoromethylation of arenes and activated aliphatic compounds such as benzyl-, allyl-, and carbonyl compounds is well studied. In contrast, introducing –CF3 groups into unactivated alkanes is considerably more challenging.
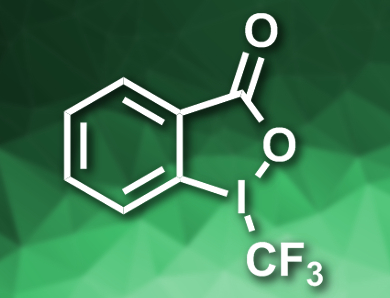
Yanchi Chen, Guobin Ma, and Hegui Gong, Shanghai University, China, have developed a cross-electrophile coupling between an alkyl electrophile and an electrophilic CF3 species under mild conditions. The team used alkyl iodides as substrates, Togni’s reagent II (pictured) as a CF3 source, CuCl and NiCl2glyme (glyme = dimethoxyethane) as catalysts, pyrrolidinone as an additive, B2(nep)2/LiOMe (B2(nep)2 = bis(neopentyl glycolato)diboron) as a reductant, and dimethylformamide (DMF) as the solvent. The reaction gives the desired alkyl–CF3 products in moderate to good yields.
The reaction tolerates a variety of functional groups and has a broad substrate scope. The researchers propose a reaction mechanism involving both alkyl and CF3 radicals, with the reaction of an alkyl–Cu complex with Togni’s reagent as a key step. According to the team, the reaction represents one of the first catalytic processes for the cross-electrophile trifluoromethylation of alkyl electrophiles.
- Copper-Catalyzed Reductive Trifluoromethylation of Alkyl Iodides with Togni’s Reagent,
Yanchi Chen, Guobin Ma, Hegui Gong,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02005