Compounds with many nitrogen–nitrogen bonds can often be useful as energetic materials. However, they are usually unstable under ambient conditions and can, thus, be difficult to synthesize. One approach to stabilizing such compounds is the use of high pressures.
Maxim Bykov, University of Bayreuth, Germany, and colleagues have synthesized three new compounds in the iron-nitrogen system, Fe3N2, FeN2. and FeN4. The team used a direct reaction between an Fe foil and N2 in a laser-heated diamond anvil cell. Fe3N2 was synthesized at 50 GPa, FeN2 at 58 GPa, and FeN4 at 106–135 GPa. The reaction products were characterized using single-crystal X-ray diffraction.
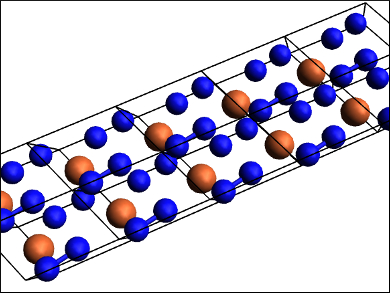
Fe3N2 is isostructural to the chromium carbide Cr3C2. FeN2 has a marcasite (FeS2) structure type. FeN4 (structure pictured) features polymeric nitrogen chains of the type [N42−]n. While the high pressure needed for the synthesis of FeN4 makes it impractical for applications as a high-energy material, its estimated volumetric energy density is higher than that of TNT (2,4,6-trinitrotoluene) or RDX (1,3,5-trinitroperhydro-1,3,5-triazine). According to the researchers, this first characterized poly-nitrogen compound could be important for further theoretical and experimental studies in the field.
- Fe-N system at high pressure reveals a compound featuring polymeric nitrogen chains,
M. Bykov, E. Bykova, G. Aprilis, K. Glazyrin, E. Koemets, I. Chuvashova, I. Kupenko, C. McCammon, M. Mezouar, V. Prakapenka, H.-P. Liermann, F. Tasnádi, A. V. Ponomareva, I. A. Abrikosov, N. Dubrovinskaia, L. Dubrovinsky,
Nat. Commun. 2018.
https://doi.org/10.1038/s41467-018-05143-2