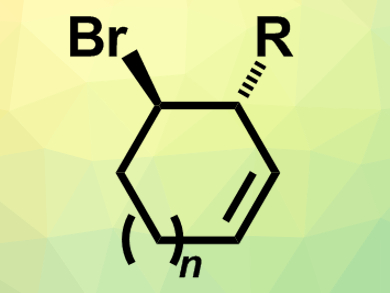
Meso-compounds contain stereogenic centers, but are symmetrical overall and, thus, achiral. Reactions that break this symmetry are useful for the synthesis of chiral compounds because several stereocenters can be easily formed in one step.
Ben L. Feringa, University of Groningen, The Netherlands, and colleagues have developed such a desymmetrization reaction that converts meso-dibromocycloalkenes into chiral bromocycloalkenes (pictured). The team reacted meso-1,4-dibromocycloalk-2-enes with organolithium compounds (RLi) in the presence of a copper(I)-based catalyst with a chiral phosphoramidite ligand. The resulting asymmetric allylic substitution reaction converts the substrates into the corresponding enantioenriched substituted bromocycloalkenes.
The reaction proceeds in good yields and with high regio- and enantioselectivity (enantiomeric ratio up to 99:1). The approach can be used to generate five- and six-membered bromocycloalkenes. If seven-membered substrates are used, a ring contraction can occur during purification and the reaction leads to substituted cyclohexenes in a stereospecific manner. According to the researchers, the products can, e.g., be used for the synthesis of chiral cyclic amino alcohols, which occur in natural products and bioactive compounds.
- Desymmetrization of meso-Dibromocycloalkenes through Copper(I)-Catalyzed Asymmetric Allylic Substitution with Organolithium Reagents,
Shermin S. Goh, Sureshbabu Guduguntla, Takashi Kikuchi, Martin Lutz, Edwin Otten, Makoto Fujita, Ben L. Feringa,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b02992