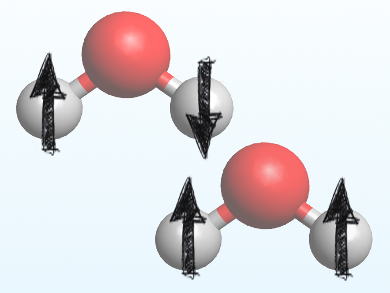
Water exists in two different forms, determined by the spin of the hydrogen atoms’ nuclei: para-water (pictured top) with opposing nuclear spins and ortho-water (pictured bottom) with the same nuclear spins. Both isomers are in equilibrium with each other. Because both isomers have almost the same physical properties, they are extremely difficult to separate. This, in turn, makes it difficult to know if and how their presence affects chemical reactions.
Ardita Kilaj, University of Basel, Switzerland, and colleagues have passed a stream of extremely cool water molecules in a vacuum chamber through an electric field. Depending on the spin symmetry of the hydrogen atoms, the H2O molecules are deflected at a slightly different angle and the isomers thus form two separate beams. The scientists led these molecular beams into an ion trap in which the ortho– and para-water each reacted with ultracold diazenylium ions.
The para-water reacted about 23 % faster than ortho-water. In addition, additional model calculations showed that the energy curves of the molecules in this reaction decreased more slowly in ortho-water than in para-water.
The researchers explain this by saying that the nuclear spin of the two hydrogen atoms influences the angular momentum of the molecule. This influences how H2O and the reaction partner diazenylium meet and leads to different degrees of attraction between them. Para-water is able to attract its reactant more strongly than the ortho-form, which results in an increased chemical reactivity.
- Observation of different reactivities of para and ortho-water towards trapped diazenylium ions,
Ardita Kilaj, Hong Gao, Daniel Rösch, Uxia Rivero, Jochen Küpper, Stefan Willitsch,
Nat. Commun. 2018.
https://doi.org/10.1038/s41467-018-04483-3