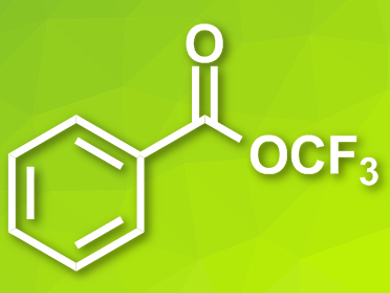
Trifluoromethoxy groups (–OCF3) are common, e.g., in potent bioactive compounds. However, introducing the group into compounds is often difficult due to the fact that known trifluoromethoxylation reagents can be toxic, volatile, expensive, or not very reactive.
Jinbo Hu and colleagues, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, have developed an inexpensive, easy-to-synthesize, shelf-stable, and efficient alternative to these reagents, trifluoromethyl benzoate (TFBz, pictured). The team treated triphosgene with KF and a catalytic amount of 18-crown-6 in acetonitrile to produce COF2. 18-Crown-6 is a crown ether that can bind potassium ions and, thus, make the fluoride in KF more reactive. The generated COF2 was again reacted with KF/18-crown-6 in tetrahydrofuran (THF), and benzoyl bromide was added to give the desired TFBz as a stable liquid with a yield of 70 %.
The prepared TFBz can be activated by a fluoride anion and releases a CF3O− anion. This anion can then react with a variety of compounds. For example, the researchers successfully used TFBz for the trifluoromethoxylation−halogenation of arynes, for nucleophilic substitutions at alkyl halides, and for the asymmetric difunctionalization of alkenes.
- Trifluoromethyl Benzoate: A Versatile Trifluoromethoxylation Reagent,
Min Zhou, Chuanfa Ni, Yuwen Zeng, Jinbo Hu,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b04000