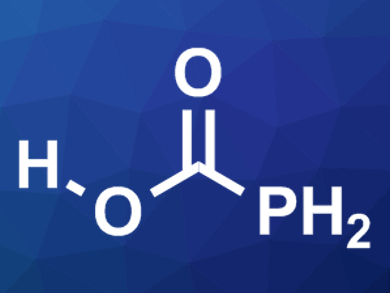
The differences between analogous nitrogen and phosphorus compounds are interesting for the study of chemical bonds and reactivities. The preference of nitrogen for double bonds often makes for more stable compounds. Carbamic acid (H2NCOOH), for example, has been synthesized in low-temperature matrices. In contrast, its phosphorus analogue, phosphino formic acid (H2PCOOH, pictured), had not been detected so far without stabilizing groups.
Ralf I. Kaiser, University of Hawaii at Mānoa, Honolulu, USA, and colleagues have detected free phosphino formic acid for the first time. The team placed mixtures of phosphine (PH3) and carbon dioxide ices in an ultrahigh vacuum and initiated reactions by exposing the ices to energetic electrons. The samples were then warmed slowly and the evaporating reaction products were detected using mass spectrometry (MS).
Quantum chemical calculations showed that H2PCOOH has an unexpectedly high kinetic stability, with a barrier of 292 kJ/mol for the dissociation into PH3 and CO2. According to the team, the synthesis proceeds via the formation of radicals and a recombination of the HOCO and PH2 fragments.
- First identification of unstable phosphino formic acid (H2PCOOH),
Cheng Zhu, Robert Frigge, Andrew Martin Turner, Ralf I. Kaiser, Bing-Jian Sun, Si-Ying Chen, Agnes H. H. Chang,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc01391h