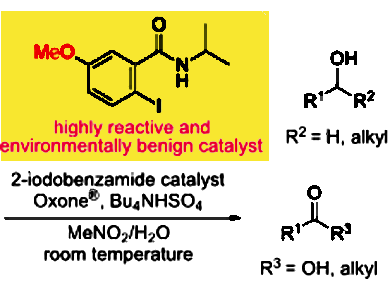
Oxidation is a fundamental and frequently used transformation in organic synthesis. Therefore, replacing the heavy metal-based catalysts which have been extensively used for a long time, is of great interest to develop efficient and environmentally benign organic synthesis.
Takayuki Yakura, University of Toyama, Japan, and colleagues have developed 2-iodo-N-isopropyl-5-methoxybenzamide as an efficient catalyst for the oxidation of primary and secondary alcohols. The reaction of benzylic and aliphatic alcohols with a catalytic amount of the catalyst in the presence of Oxone® (2KHSO5·KHSO4·K2SO4) as a co-oxidant and Bu4NHSO4 at room temperature gave good to excellent yields of the corresponding carbonyl compounds.
The team had evaluated several N-isopropyliodobenzamides as catalysts for the oxidation of benzhydrol to benzophenone. A study on the substituent effect of the benzene ring on the oxidation showed that its reactivity increased in the following order of substitution: 5-NO2 < 5-CO2Me, 3-OMe < 5-OAc < 5-Cl < H, 4-OMe < 5-Me < 5-OMe. The high reactivity of the 5-methoxy derivative at room temperature is a result of the rapid generation of the pentavalent species from the trivalent species during the oxidation.
According to the researchers, 5-methoxy-2-iodobenzamide is an efficient and environmentally benign catalyst for the oxidation of alcohols, especially benzylic alcohols.
- 2-Iodo-N-isopropyl-5-methoxybenzamide as a highly reactive and environmentally benign catalyst for alcohol oxidation,
Takayuki Yakura, Tomoya Fujiwara, Akihiro Yamada, Hisanori Nambu,
Beilstein J. Org. Chem. 2018, 14, 971–978.
https://doi.org/10.3762/bjoc.14.82
This article is part of the Thematic Series Hypervalent iodine chemistry in organic synthesis. Guest Editor is Thomas Wirth, Cardiff University, UK