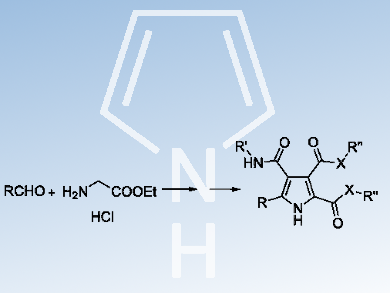
Dongfeng Zhang, Haihong Huang, and colleagues, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China, have developed a straightforward, one-pot synthesis of pyrrolo[3,4-c]pyrrole-1,3-diones. The reaction occurs via Ag(I)-catalyzed 1,3-dipolar cycloaddition of azomethine ylides from diverse commercially available aldehydes with N-alkyl maleimide, followed by readily complete oxidation with DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) as an oxidant. Further transformation with alkylamine/sodium alkoxide alcohol solution conveniently led to novel polysubstituted pyrroles in good to excellent yields.

According to the researchers, this synthesis of highly functionalized pyrroles performed well over a broad scope of substrates. They hope that this efficient construction method for privileged pyrrole scaffolds could deliver more active compounds for medicinal chemistry research. Further research on the development of more diversified pyrrolo[3,4-c]pyrrole-1,3-diones and more complicated polysubstituted pyrroles is currently underway.
- An efficient and facile access to highly functionalized pyrrole derivatives,
Meng Gao, Wenting Zhao, Hongyi Zhao, Ziyun Lin, Dongfeng Zhang, Haihong Huang,
Beilstein J. Org. Chem. 2018, 14, 884–890.
https://doi.org/10.3762/bjoc.14.75