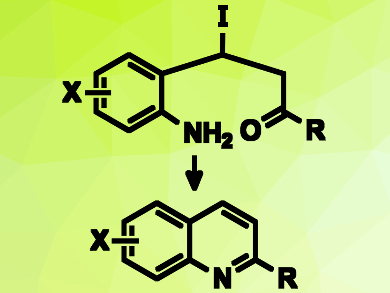
Substituted quinolines are common chemical frameworks in bioactive compounds and useful building blocks in materials science. They could potentially be synthesized by a cyclization of 2-aminostyryl ketone derivatives. However, the more stable (E) configuration these styryls prevents close contact between the anime and the carbonyl group, and thus, the cyclization.
Cheol-Hong Cheon and colleagues, Korea University, Seoul, have used iodide as a catalyst to allow this type of reaction. The team combined 2-aminostyryl ketones with tetrabutylammonium iodide (TBAI) as a nucleophilic catalyst. The iodide is added to the styryl double bond, and the resulting β-iodoketones can adopt a cis-like conformation (pictured top). The amino and carbonyl groups can then undergo a condensation reaction to form a ring. A subsequent elimination of hydrogen iodide provides the desired quinolines (pictured bottom) and regenerates the catalyst.
The reaction is suitable for a variety of 2-aminostyryl ketone substrates and proceeds in excellent yields. In addition, the resulting quinolines can be further transformed without isolation of the product.
- Synthesis of 2-Substituted Quinolines from 2-Aminostyryl Ketones Using Iodide as a Catalyst,
So Young Lee, Jiye Jeon, Cheol-Hong Cheon,
J. Org. Chem. 2018.
https://doi.org/10.1021/acs.joc.8b00552