Due to an increasing demand for clean, renewable energy, technologies such as hydrogen and oxygen production from water splitting and rechargeable metal-air batteries have garnered significant interest. However, the underlying chemical processes of these technologies—i.e., the oxygen evolution reaction (OER), the oxygen reduction reaction (ORR), and the hydrogen evolution reaction (HER)—suffer from slow kinetics and often require expensive noble metal catalysts.
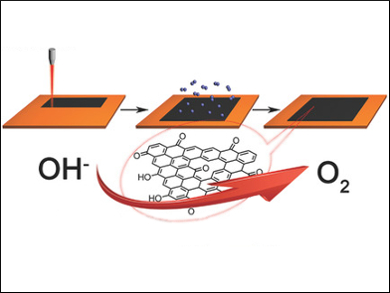
Boris J. Yakobson, James M. Tour, Rice University, Houston, USA, and colleagues have developed an efficient metal-free catalyst for OER and ORR based on laser-induced graphene (LIG), a 3D porous graphene material. The LIG is prepared by a one-step laser scribing process on commercial polyimide (pictured left). The oxidation of the LIG with an O2 plasma leads to oxidized LIG (LIG-O). This enhances both the OER and ORR due to the high oxygen content (11.6 %), which provides active sites for electrocatalysis.
The OER with this LIG-O material showed a remarkably lowered onset potential of 260 mV with a low Tafel slope of 49 mV dec–1. This is lower than most other metal-free catalysts. The team also examined the electrocatalytic activity of LIG-O in a Li-O2 battery. The overpotential upon charging was decreased from 1.01 V in LIG to 0.63 V in LIG-O. This can be attributed to the oxygen-containing groups facilitating the adsorption of OER intermediates and lowering the activation energy.
- Oxidized Laser-Induced Graphene for Efficient Oxygen Electrocatalysis,
Jibo Zhang, Muqing Ren, Luqing Wang, Yilun Li, Boris I. Yakobson, James M. Tour,
Adv. Mater. 2018.
https://doi.org/10.1002/adma.201707319