Nitrogenases catalyze the reduction of N2 to NH3 at the expense of ATP-hydrolysis. All nitrogenases are a bi-enzyme cascade. It consist of a reducing component protein (Fe protein) and one of three catalytic proteins. These are characterized by their molybdenum−iron (MoFe)-, vanadium−iron (VFe)-, or iron-only (FeFe) catalytic co-factors. MoFe nitrogenase is the “conventional” nitrogenase. VFe nitrogenase (VFe) and Fe nitrogenase (FeFe) are alternative nitrogenases that are expressed under Mo starvation.
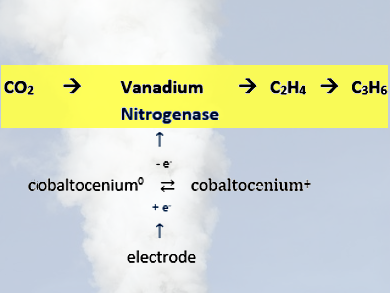
Shelley D. Minteer, Ross D. Milton, and colleagues, University of Utah, Salt Lake City, USA, have developed a bioelectrocatalysis of vanadium nitrogenase (VFe) from Azotobacter vinelandii (pictured). The bioelectrocatalytic VFe system reduces CO2 to ethylene and propene, bypassing the requirement of CO as the substrate and forming C−C bonds. In the biocatalysis, cobaltocenium derivatives transfer electrons to the catalytic VFe protein, independent of ATP-hydrolysis.
According to the researchers, this bioelectrocatalytic approach will be essential in revealing further mechanistic details that may lead to improved biological and molecular electrocatalysts for CO2RRs and C−C bond formation.
- Electroenzymatic C–C Bond Formation from CO2,
Rong Cai, Ross D. Milton, Sofiene Abdellaoui, Terry Park, Janki Patel, Bassam Alkotaini, Shelley D. Minteer
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b02319