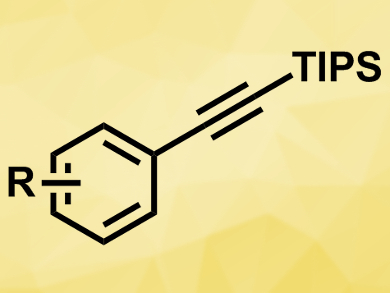
Alkynes are useful intermediates in organic synthesis. Often, alkynes are prepared using coupling reactions with organometallic nucleophiles. However, these strong nucleophiles limit the reactions’ functional group tolerance.
Kosuke Yasui, Naoto Chatani, and Mamoru Tobisu, Osaka University, Japan, have developed a rhodium-catalyzed alkynylation of aryl carbamates that avoids this problem by using propargyl alcohols as the alkynylating agent. The team combined a range of aryl carbamates with triisopropylsilyl ether (TIPS)-protected propargyl alcohols using [RhCl(C2H4)2] as a catalyst, an N-heterocyclic carbene (NHC) as a ligand and K3PO4 as a base in toluene at 130 °C.
The reaction gives the desired aryl acetylenes (pictured) in good yields and tolerates functional groups such as ketones, esters, and amides. It proceeds via C−O bond activation at the carbamate, which acts as an ortho-directing group. According to the researchers, the synthesized functionalized aromatic alkynes can serve as useful building blocks in organic synthesis.
- Rhodium-Catalyzed C–O Bond Alkynylation of Aryl Carbamates with Propargyl Alcohols,
Kosuke Yasui, Naoto Chatani, Mamoru Tobisu,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b00674