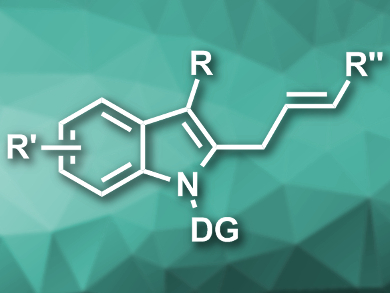
Allyl arenes are useful synthetic intermediates. Their synthesis usually involves methods with limited substrate scopes and/or harsh reaction conditions.
Xiaowei Wu, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA, Haitao Ji, H. Lee Moffitt Cancer Center and Research Institute and University of South Florida, Tampa, USA, have developed a ruthenium-catalyzed allylation of indoles under mild conditions that tolerates a range of functional groups. The team used [{RuCl2(p-cymene)}2] as a catalyst and sodium acetate as a base to couple a variety of indoles featuring an N-ethoxycarbamoyl directing group with allyl alcohols in methanol at 45 °C (example product pictured).
The reaction proceeds via a β-hydroxide elimination and has good to excellent regio- and stereoselectivity. The method tolerates both electron-donating and electron-withdrawing substituents at the indole and the allyl alcohol.
- Ruthenium(II)-Catalyzed Regio- and Stereoselective C–H Allylation of Indoles with Allyl Alcohols,
Xiaowei Wu, Haitao Ji,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b00567