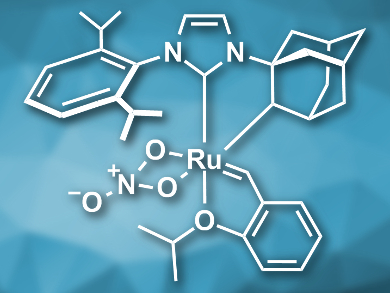
Olefin metathesis is a versatile method for the construction of C=C bonds. Ruthenium-based catalysts with N-heterocyclic carbene (NHC) ligands are very commonly used for such reactions. Unsymmetrical NHC ligands can be particularly useful for Z-selective metathesis reactions, but they can be difficult to synthesize on a large scale and, thus, too expensive for industrial use.
Olivier Baslé, Marc Mauduit, Ecole Nationale Superieure de Chimie de Rennes, CNRS, France, and colleagues have synthesized a Z-selective metathesis catalyst (pictured) on a multigram scale in good yields using a multicomponent synthesis. The team combined 2,6‐diisopropylaniline, glyoxal, formaldehyde, and adamantylamine to prepare the imidazolium precursor of the NHC ligand.
The imidazolium precursor was deprotonated using potassium bis(trimethylsilyl)amide (KHMDS) to give the NHC ligand. This ligand was then reacted with the first generation Hoveyda−Grubbs catalyst and finally converted to the desired catalyst by C-H bond activation at the adamantyl group and the addition of a nitrato ligand. The product was obtained in an overall yield of 43 %.
The developed catalyst shows excellent activity for olefin metathesis, high Z-selectivity, and high conversion rates. It could also be used in ring-opening metathesis polymerization for the controlled synthesis of norbornene-, norbornadiene-, and cyclopropene-based polymers.
- A Versatile and Highly Z-Selective Olefin Metathesis Ruthenium Catalyst Based on a Readily Accessible N-Heterocyclic Carbene,
Adrien Dumas, Robert Tarrieu, Thomas Vives, Thierry Roisnel, Vincent Dorcet, Olivier Baslé, Marc Mauduit,
ACS Catal. 2018.
https://doi.org/10.1021/acscatal.8b00151