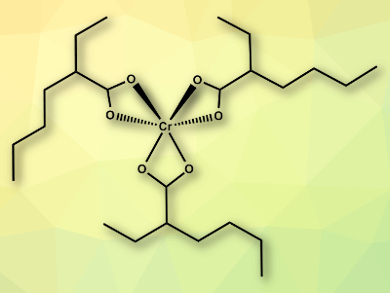
Chromium(III) carboxylate complexes have a wide variety of structures, from dimers up to clusters with many chromium centers. The complexes have applications in catalysis and materials science. However, complexes with multiple chromium atoms often contain coordinated water or acid molecules, which can hamper catalytic applications.
Orson L. Sydora, Chevron Phillips Chemical LP, Kingwood, TX, USA, and colleagues have synthesized the first homoleptic monomeric chromium(III) carboxylate complex Cr(EH)3 (EH = 2-ethylhexanoate; complex pictured). The team combined sodium 2-ethylhexanoate (NaEH) in tetrahydrofuran (THF) with CrCl3(THF)3. Anhydrous salt metathesis then causes NaCl to precipitate and the desired Cr(III) complex is formed.
The team characterized the complex using elemental analysis, infrared (IR) and Raman spectroscopy, Field Desorption Mass Spectrometry (FD-MS), and high-energy X-ray diffraction. They also studied the structure using density functional theory (DFT) calculations. The complex is soluble in organic solvents and is water- and acid-free, which could make it suitable for catalytic applications.
- A homoleptic chromium(III) carboxylate,
O. L. Sydora, R. T. Hart, N. A. Eckert, E. Martinez Baez, A. E. Clark, C. J. Benmore,
Dalton Trans. 2018.
https://doi.org/10.1039/c8dt00029h