While there are many iron nitrides, none with an N/Fe ratio larger than one had been synthesized so far. Pernitrides of the type MN2 with other transition metals had been prepared under high pressures and temperatures. Theoretical calculations predict that the iron pernitride FeN2 should be stable under pressure.
Dominique Laniel, Commissariat à l’énergie atomique et aux énergies alternatives (CEA), Direction des applications militaires Île-de-France (DIF), Arpajon, France, and colleagues have synthesized and characterized FeN2. The team used laser-heated diamond anvil cells to compress iron powder under a nitrogen atmosphere and obtained the pernitride at pressures above 72 GPa and temperatures above 2200 K. The reaction was monitored using powder X-ray diffraction and Raman spectroscopy.
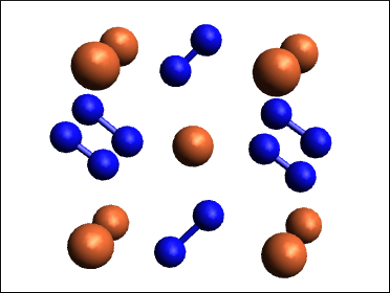
FeN2 has a marcasite-type structure (pictured, N in blue, Fe in dark orange).The bond order in the nitrogen dimers was determined to be about 1.5 based on the lattice parameters and Raman frequencies. The compound has a bulk modulus (a measure of resistance to compression) of 344 GPa, which represents a more than 200 % increase over that of pure iron.
- High Pressure and High Temperature Synthesis of the Iron Pernitride FeN2,
Dominique Laniel, Agnès Dewaele, Gaston Garbarino,
Inorg. Chem. 2018.
https://doi.org/10.1021/acs.inorgchem.7b03272