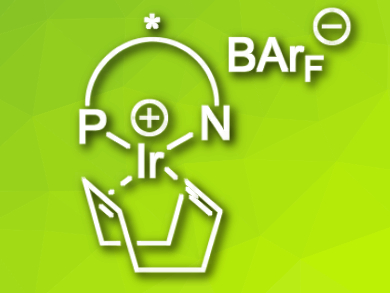
Chiral iridium complexes with N,P ligands are highly effective catalysts for asymmetric hydrogenation reactions. However, they are expensive for large-scale reactions and sometimes need high catalyst loadings. Although catalyst recovery is an active research field, the recovery of Ir catalysts has rarely been explored due to the instability of the catalytically active Ir complexes in the reaction medium.
Andreas Pfalz, University of Basel, Switzerland, and colleagues have developed a practical and simple protocol to recover Ir (N,P ligand) catalysts as stable COD [1,5-cyclooctadiene] complexes (pictured) from asymmetric hydrogenation reactions. The researchers used Ir(N,P ligand)(COD) complexes as precatalysts. The active species resulting from the reaction of this precatalyst with dihydrogen are too reactive to be isolated after use. Instead, the team added COD again at the end of the reaction, followed by a usual workup and chromatography. The more stable precatalysts could, thus, be recovered in 60–70 % yields with high purity.
The recovered catalysts show almost the same reactivity and enantioselectivity in asymmetric hydrogenation reactions as the original ones. Using NMR studies, the researchers found that dimeric Ir(III) dihydride complexes were formed after the reaction and COD could react with these dimers to regenerate the original precatalysts.
- Recovery and Recycling of Chiral Iridium(N,P Ligand) Catalysts from Hydrogenation Reactions,
Marc-André Müller, Stefan Gruber, Andreas Pfaltz,
Adv. Synth. Catal. 2018.
https://doi.org/10.1002/adsc.201701591