Chelating ligands are useful for building metal-based catalysts. However, the synthesis of, e.g., functionalized diphosphine ligands can be difficult. Using self-assembly, researchers can use monophosphines instead and arrange two of them around a metal atom.
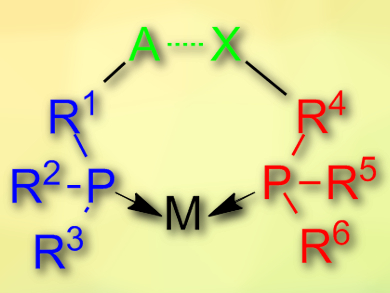
Piet W. N. M. van Leeuwen, l’Institut National des Sciences Appliquées de Toulouse (INSA), France, Anton Vidal-Ferran, Institute of Chemical Research of Catalonia (ICIQ) and Barcelona Institute of Science and Technology, Tarragona, Spain, and Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain, and colleagues have, for the first time, used halogen bonding as a tool to assemble such catalysts. The team used two different phosphines, one with a pyridyl substituent that acts as a halogen-bond acceptor (A in the picture), and one with a iodotetrafluoroaryl substituent that acts as a halogen-bond donor (X in the picture). They combined these two ligands with [Rh(Cl)(CO)2]2 as a Rh(I) precursor to prepare the final catalyst.
The two phosphines in the catalyst form a halogen bond (pictured) and simulate the effect of a chelating diphosphine ligand. The catalyst was used for the hydroboration of terminal alkynes. Its catalytic activity is comparable to the highest-performing catalysts to date. In addition, the prepared catalyst allows access to previously difficult to synthesize branched alkenyl boronic acids.
- XBphos-Rh: A Halogen-Bond Assembled Supramolecular Catalyst,
Anton Vidal-Ferran, Lucas Carreras Vinent, Marta Serrano Torne, Piet W.N.M. van Leeuwen,
Chem. Sci. 2018.
https://doi.org/10.1039/c8sc00233a