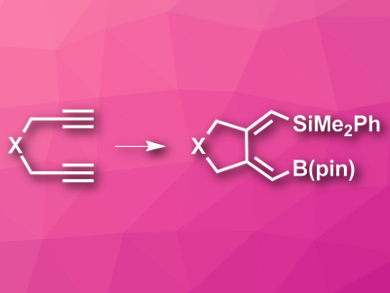
The combination of addition and cyclization reactions allows the synthesis of useful highly substituted cyclic compounds in one step. For example, palladium-catalyzed reactions of this type can be used to introduce stannyl, boryl, or silyl groups into the products.
Yun-He Xu, University of Science and Technology of China, Hefei, and colleagues have developed a protocol for the conversion of 1,6-diynes to 1,2-dialkylidenecycloalkanes with silyl and boryl groups. The team reacted (dimethylphenylsilyl)pinacolborane, PhMe2Si-B(pin) (pin = (CH3)4C2O2), as a silaboration reagent with different 1,6-diynes. Pd2(dba)3 (dba = dibenzylideneacetone) was used as the catalyst, and the reactions were performed in tetrahydrofuran THF at 50 °C.
The reaction gives the desired substituted cycloalkanes in a (Z,Z)-configuration in good to excellent yields. The approach can be used for a variety of substrates and tolerates a wide range of functional groups. According to the researchers, the products can be easily converted to other useful synthetic intermediates.
- Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes,
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu, Teck-Peng Loh,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc00097b