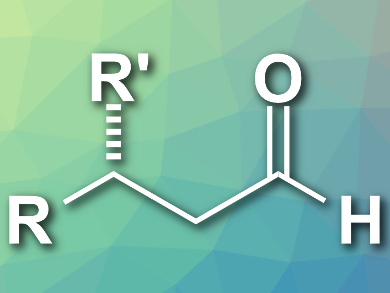
Aldehydes with a β-stereogenic center are important building blocks in organic synthesis. The direct conversion of α,β-unsaturated carboxylic acids to β-chiral aldehydes (example pictured) is an attractive approach because the starting materials are stable and readily available.
Stephen L. Buchwald and colleagues, Massachusetts Institute of Technology (MIT), Cambridge, USA, have developed an efficient asymmetric reduction process to synthesize β-chiral aldehydes from unsaturated carboxylic acids using Cu(OAc)2 as a catalyst, 1,2-bis((2S,5S)2,5-diphenylphospholano)ethane as a ligand, and dimethoxymethylsilane as a reductant. The reactions are carried out in toluene at 40 °C over 18 h and quenched with (NH)4F in MeOH.
The approach gives the desired chiral aldehydes in good yields with high enantioselectivity and broad functional group tolerance. The researchers propose a ketene intermediate based on mechanistic studies and density functional theory (DFT) calculations. They also demonstrated the synthetic utility of this transformation by synthesizing γ-chiral amines from the unsaturated carboxylic acids via a one-pot reductive amination reaction.
- CuH-Catalyzed Asymmetric Reduction of α,β-Unsaturated Carboxylic Acids to β-Chiral Aldehydes,
Yujing Zhou, Jeffrey S. Bandar, Richard Y. Liu, Stephen L. Buchwald,
J. Am. Chem. Soc. 2018, 140, 606–609.
https://doi.org/10.1021/jacs.7b12260
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)


