Aerobic oxidations using Pd catalysts have been known for decades. The interest in them has been renewed by the discovery that Pd(0) can be readily reoxidized by O2 in appropriate ligation environments. While air or O2 is a convenient and readily available oxidant, partially reduced O2 species can deactivate the catalyst via oxidative ligand degradation. Another typical catalyst deactivation mechanism that limits the lifetime of Pd catalysts is the formation of Pd black.
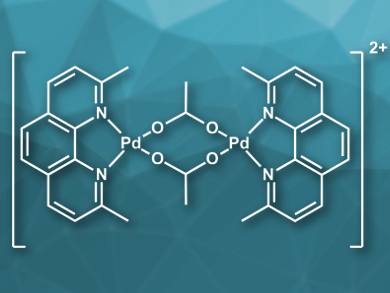
Robert M. Waymouth, Standford University, CA, USA, and colleagues have studied several Pd-catalyzed selective oxidations using O2 as the terminal oxidant with the palladium complex [(neocuproine)Pd(μ-OAc)]2[OTf]2 (cation pictured, neocuproine = 2,9-dimethyl-1,10-phenanthroline, Tf = trifluoromethanesulfonate) as the catalyst. Mechanistic studies were performed to identify catalyst deactivation pathways and several strategies have been developed to improve catalyst lifetimes and turnover numbers.
The researchers found that oxidative ligand degradation could be suppressed by ligand modification, addition of phenolic additives, and addition of benzylic hydroperoxides or styrene. They also found that styrene could prevent the formation of Pd black by intercepting Pd hydrides. These strategies allow the catalyst loading to be reduced from 5–10 mol% to 0.25 mol%.
- Pd-Catalyzed Aerobic Oxidation Reactions: Strategies To Increase Catalyst Lifetimes,
Wilson C. Ho, Kevin Chung, Andrew J. Ingram, Robert M. Waymouth,
J. Am. Chem. Soc. 2018, 140, 748–757.
https://doi.org/10.1021/jacs.7b11372