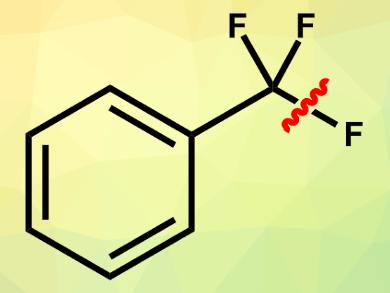
Aryldifluoromethyl derivatives are building blocks of many bioactive compounds. The selective single C(sp3)–F bond cleavage of easily available trifluoromethylarenes could be a straightforward method to synthesize such compounds. However, avoiding multiple defluorinations is very challenging.
Ruth Gschwind, Burkhard König, University of Regensburg, Germany, and colleagues have found an efficient way to selectively cleave a single C–F bond in trimethylarenes by using fac-Ir(ppy)3 as a photocatalyst under blue-light irradiation. A Lewis acidic fluoride scavenger, pinacolborane, and a base, 2,2,6,6-tetramethylpiperidine, are used to activate the reaction and improve the efficiency. The reactions are carried out in 1,2-dichloroethane at 20 °C.
N-Methyl-N-phenylmethacrylamide was chosen as a trapping reagent to capture the produced aryldifluoromethyl radical. A subsequent cyclization and oxidative rearomatization give the desired difluoroarene products. The reaction tolerates a range of functional groups. Mechanistic studies show that the chemoselectivity of single C–F bond cleavage is controlled by both steric and electronic factors of the Lewis acid and the base.
- Selective Single C(sp3)–F Bond Cleavage in Trifluoromethylarenes: Merging Visible-Light Catalysis with Lewis Acid Activation,
Kang Chen, Nele Berg, Ruth Gschwind, Burkhard König,
J. Am. Chem. Soc. 2017, 139, 18444–18447.
https://doi.org/10.1021/jacs.7b10755